Hydrogen from Natural Gas without Carbon Dioxide Emissions
Mark Robertson, PE., LEC Partners
Abstract: Thermo-catalytic decomposition of methane (TCDM) converts methane directly into hydrogen and a useful solid carbon byproduct, with reduced greenhouse gas emissions. The resultant (turquoise) hydrogen can be used as a fuel in power generation, heating, transportation and for industrial hydrogen use. The solid carbon byproduct, in the form of carbon nanotubes, is an extremely lightweight and strong material that can replace or be added to existing materials, to provide performance improvements with a significant reduction in overall greenhouse gas emissions in the production and use of the materials.
Introduction:
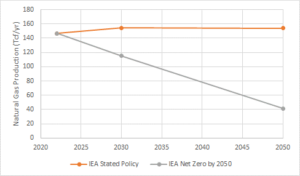
The uncertainty in the future for natural gas in our energy portfolio is best illustrated by the divergent projections generated for natural gas production in various “business as usual” scenarios and more ambitious carbon-constrained scenarios. For example, Figure 1 illustrates the projections for global natural gas production in the International Energy Agency (IEA, World Energy Outlook, 2022) Stated Policies Scenario, which represents our current climate trajectory (i.e. business as usual) and the IEA Net Zero by 2050 Scenario, which targets an increased global average temperature increase below 2 oC above pre-industrial levels.
The world’s proven natural gas reserves are sufficient to last approximately 50 years, at current consumption rates (Worldometer, 2020), significantly exceeding both of the carbon-constrained production projections described above. These trends, in conjunction with recent regulatory pressures too, for example, limit the use of natural gas in new building construction, indicating there is a real possibility that a significant percentage of our proven natural gas reserves will need to be left in the ground to meet climate change goals. Natural gas industry stakeholders need to recognize that natural gas’ future as a transitional energy source is uncertain and the industry should support research and development aimed at more sustainable natural gas production and use.
Methane Combustion and Carbon Capture:
The combustion of methane, which is the predominant molecule present in natural gas, is represented by the following exothermic chemical reaction:
Continued natural gas production and consumption in a carbon-constrained energy future is most often discussed in conjunction with so-called carbon capture and sequestration (CCS), where carbon dioxide produced from natural gas combustion is captured and stored in deep underground geologic formations. A limited number of CCS projects may be economic at carbon pricing of $50/ton CO2, but large centralized natural gas-fueled power stations probably require carbon pricing over $100/ton CO2 for CCS to be economic more broadly (Rubin Edward S., 2012 ).
Carbon capture and utilization (CCU) also requires carbon capture but instead of sequestering the captured CO2 underground, the carbon dioxide is used as a feedstock in the production of useful products, fuels or chemicals. As these processes require substantial energy input to facilitate the conversion of zero energy content CO2 into other useful products, they often require access to large quantities of renewable power and/or energy and typically require carbon pricing of well over $100/ton CO2 to be economic (Bazzanella A.M., Ausfelder F., 2017).
CCS and CCU are attracting significant research and development and project funding. The NETL Carbon Capture and Storage Database (NETL, 2020) lists 42 completed or active projects, of capacity over 1,000 metric tonnes per day of CO2, with a total capital cost exceeding $12 billion US dollars. The European Union’s Innovation Fund plans to make up to 10 billion Euros available before 2030, in large part for CCS and CCU projects.
CCS and CCU will be deployed more widely in our future energy systems, but there is a significant risk that the deployment will be hindered by insufficient carbon prices and/or other credits or incentives required to make the technologies viable and in other cases, CCS and/or CCU may not be technically feasible in a specific market segment, industry or at particular geographic locations.
Hence, there is a need for alternate technology pathways that would also allow for the utilization of our natural gas resources in a more sustainable manner, particularly where the CO2 mitigation costs are lower than for other alternative technology pathways.
Methane Decomposition and Hydrogen Generation
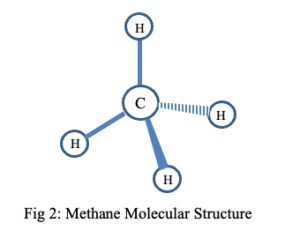
The molecular structure of methane is illustrated below (See Fig 2).
n the presence of a suitable catalyst, the carbon-hydrogen bonds in the methane can be broken at a relatively low reaction temperature (<1250 oF), resulting in the production of molecular hydrogen and a solid carbon byproduct. The endothermic reaction is represented as:
The (turquoise) hydrogen produced by this reaction can be combusted as a fuel for heat, oxidized in a fuel cell to produce power or used in industrial processes. The exothermic hydrogen oxidation reaction is represented as:
Reaction 2 & 3 can be combined into a single exothermic reaction:
Reaction 4 will produce a net energy release of 463 Btu per standard cubic foot of methane as compared to an energy release of 909 Btu per standard cubic foot, if the methane was simply combusted, as per Reaction 1. Hence, at first glance, the combination of Reaction 2+3 (i.e. Reaction 4) would appear to represent a significant loss of energy yield per unit of methane (or natural gas) consumed. However, Reaction 2 makes the usable energy available in the form of molecular hydrogen. Hydrogen has some significant benefits as an energy source over methane one of which is that it can be utilized directly in fuel cells for the production of electricity. A hydrogen fuel cell in a stationary power application can be 60% efficient (US DOE, 2006), meaning that 60% of the energy generated from the hydrogen oxidation in the fuel cell is converted to electrical energy, not taking into account additional useful byproduct heat generation. For existing natural gas power plants, the average plant electrical efficiency is only 36% (IEA, Key World Energy Statistics, 2019).
The key feature of Reaction 4 which reduces greenhouse gas (GHG) emissions is that carbon present in the original methane molecule is not oxidized to produce a gaseous CO2 byproduct, as is the case for methane combustion (Reaction 1), but instead, the carbon is converted to a solid byproduct, which is not subsequently released into the atmosphere. Hence the carbon atoms in the methane molecule are sequestered in the solid carbon byproduct.
Byproduct Carbon Nanotubes (CNTs):
Carbon can exist in an amorphous (shapeless) form such as charcoal. It can also be present in the form of graphite, which is a crystalline form of carbon. In graphite, the carbon molecules are arranged in multiple layers. Graphene refers to individual layers or sheets of carbon molecules. Carbon Nanotubes (CNTs) are rolled-up sheets of graphene, forming cylinders. CNTs have special properties that make them particularly useful, namely high strength and lightweight. The strength of CNTs are approximately 100 times that of steel, but with very significantly reduced weight. In addition, CNTs are very resistant to corrosion and chemical attack.
These properties make CNTs, used in either composites or as fibers, useful in aircraft manufacture, automobiles, consumer goods, textiles and industrial products. CNTs can also be added to more conventional products and materials, to provide performance benefits. For example, CNTs can be added to concrete to provide a 20 % strength increase at very low addition levels (~0.5 wt%).
As described above, catalysts can be used to accelerate Reaction 2. These same catalyst sites can also be used to grow CNTs. The accumulation of carbon (CNTs) at these catalyst sites also poses a challenge, as the catalyst can be relatively quickly deactivated by carbon formation.
The following table represents some of the potential near-term (large volume) applications of CNTs in various end-use markets. The potential for the use of CNTs in these markets to significantly reduce GHG emissions is also illustrated.
Table 1: Potential CNT end uses
| End use for CNTs | CNT usage
Mt/yr |
CO2 Mitigated Mt/yr | CO2 Mitigated/ CNT usage t/t |
| CNT Enriched Concrete production (1) | 20 | 800 | 40 |
| Steel composites (2) | 9.6 | 2,712 | 282 |
| Aerospace (3) | 0.12 | 92 | 767 |
| Automotive (4) | 12.5 | 95 | 7.6 |
| Asphalt binders (5) | 3.3 | n.d. | n.d. |
| Carbon Black | 13 | 30 | 2.3 |
| TOTAL | 58.5 | 3,729 | 64 |
Notes:
(1). CNTs added to cement at 0.5 wt% (Ferro G., 2011), for 4 billion tons/yr production (Wang, 2020). CO2 mitigation due to 20% reduction in total cement production at 1 lb CO2 emissions / lb cement (National Ready Mixed Concrete Association, 2008).
(2). 0.75 wt% CNT steel composites, resulting in 37% higher yield strength and (assumed) 20% reduction in current steel production at 1.6 billion metric tonnes/yr (Licht S., 2019)
(3). CNTs penetration at 15 wt% of airframe structural materials (O’Donnell S.E.) of 0.8 Mtons/yr (Djukanovic, 2020). CO2 mitigation equal to 10% reduction (O’Donnell S.E.) in worldwide aviation fuel consumption of 1 billion tons/yr (Air Transport Action Group, 2019) .
(4). CNTs penetration at 5% of vehicle materials of 250 Mtons/yr. CO2 mitigation equal to 1.5% reduction in worldwide road transportation CO2emissions of 6.3 billion tons/yr.
(5). CNT added to asphalt at 3 wt% (Faizan ul Haq, 2018), for 110 Mt/yr asphalt production (Wikipedia, n.d.)
Status of Commercial Process for Catalytic Thermal Decomposition of Methane
Although hydrogen research and development is attracting high levels of funding, very little of this funding is directed toward looking for more efficient (turquoise) hydrogen generation via the thermo-catalytic decomposition of the methane pathway.
To date, the only large-scale demonstration or commercial deployment of a (supported metal) catalyzed TCDM technology has been the UOP HYPRO process (Choudhary T.V., 2006). The primary reason for the slow commercial deployment of TCDM technologies is the inability of catalyst activity to be maintained in conditions where necessary carbon formation on the catalyst surface also results in the blocking of the active sites on the catalyst surface.
To date, these challenges have not been sufficiently resolved to enable economic commercial TCDM operations, which require maintenance of high catalyst activity over extended operating runs. Hence, these issues are the most urgent to address via R&D program development.
Future Commercial TCDM Operations
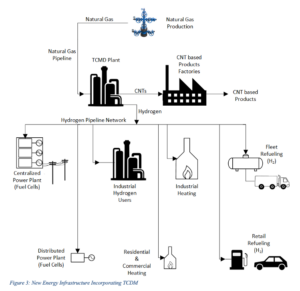
If sufficient R&D could be directed towards the challenges associated with TCDM commercial deployment, it is possible to envisage a long term future for a natural gas-based energy infrastructure based on the thermo-catalytic conversion of natural gas to hydrogen and CNTs, as represented in the following figure:
The investment in hydrogen delivery, heating and refueling systems will not need to be abandoned as natural gas production ultimately diminishes, as hydrogen from water splitting using renewable and nuclear energy would be expected to make use of this same infrastructure in the future.
For industrial hydrogen generation and industrial heating particularly, the TCDM pathway, results in significantly lower CO2 mitigation costs than other renewable energy production routes, when accounting for the GHG emission reductions associated with the effective use of the CNT byproduct.
Conclusions
Thermo-catalytic decomposition of methane (TCDM) with the productive use of the generated hydrogen and byproduct carbon nanotubes (CNTs) could provide an alternative pathway to utilize our natural gas resource, while significantly reducing the associated greenhouse gas emissions, particularly where GHG emissions associated with natural gas production and supply, including fugitive methane emissions, are controlled. R&D and commercial deployment of this technology pathway should also be pursued as a high priority.
About the Author: Mark Robertson is a registered Professional Engineer (PE) in Colorado, and has 30 years of experience in technology development, plant operations, and engineering. He is currently an expert with LEC Partners, handling matters involving gasification, biofuel and biochemical Synthesis, gas-to-liquids, biomass and waste-to-energy, techno-economics analyses and fluidization.
References:
Air Transport Action Group. (2019). facts-figures. Retrieved from atag.org: https://www.atag.org/facts-figures.html
Bazzanella A.M., Ausfelder F. (2017). Low Carbon Energy and Feedstock for European Chemical Industry. Franfurt: DECHEMA e.V.
Choudhary T.V., G. D. (2006). Methane Decomposition: Production of Hydrogen and carbon Filaments. Catalysis, Vol 19, pp. 164-183.
Djukanovic, G. (2020, April 17). Aerospace Industry Trends & Aluminium Use. Aluminium Insider.
Faizan ul Haq, M. (2018). Carbon Nanotubes in Asphalt Binder: Homogeneous Dispersion and Perfromance Enhancement. Applied Sciences, 8, 2651.
Ferro G., T. J. (2011). Carbon Nanotubes Cement Composites. Convegno Nazionale IGF XXI, (pp. 49-59). Cassino.
IEA. (2019). Key World Energy Statistics. IEA.
IEA. (2022). World Energy Outlook. Retrieved from https://www.iea.org/reports/world-energy-outlook-2022
Licht S., L. X. (2019). Amplified CO2 Reduction of Greenhouse Gas Emissions with C2CNT Carbon Nanotube Composites. Materials Today Sustainability, Vol 6.
National Ready Mixed Concrete Association. (2008). Concrete CO2 Fact Sheet. NRMCA.
NETL. (2020, May 31). Carbon Capture and Storage Database. Retrieved from https://www.netl.doe.gov/coal/carbon-storage/worldwide-ccs-database
O’Donnell S.E., S. K. (n.d.). Potential Impact of Carbon Nanotube Reinforced Polymer Composite on Commercial Aircraft Performance and Economics. American Institute of Aeronautics and Astronautics.
Rubin Edward S., Z. H. (2012 ). The Cost of Carbon Capture and Storage for Natural Gas Combined Cycle Power Plants. Environmental Science & Technology, 46 (6), 3076-3084.
US DOE. (2006). Hydrogen Fuel Cells Tact Sheet. Retrieved from www.hydrogen.energy.gov
Wang, T. (2020, Feb 13). World ans US Cement Production 2010-2019. Retrieved from Statista: https://www.statista.com/statistics/219343/cement-production-worldwide/
Wikipedia. (n.d.). Wikipedia/Asphalt. Retrieved from Wikipedia.
Worldometer. (2020). Retrieved from https://www.worldometers.info/gas/
Have some questions?
Not sure where to start?
Let's start a conversation. We're here to help you navigate
the bioeconomy with confidence.