Blue Hydrogen
Blue Hydrogen
By Terry Mazanec, Ph.D., LEC Partners
The late Sir John Cadogan, renowned organic chemist and Welsh Member of Academia Europaea, was the first person whom I heard exclaim that we were on a path towards the ‘hydrogen economy.’ It was 1986, and my company, SOHIO (The Standard Oil Company (Ohio)), was just about to merge with British Petroleum (BP), where Cadogan was the worldwide Director of Research. Sir John presented the goals and plans for the combined BP and SOHIO research organizations to the assembled R&D staff of SOHIO. The hydrogen economy, he assured us, was not far in the future, but within reach ‘in a few short years.’ This was music to my ears since I was deeply involved in research on hydrogen production technologies. Some 37 years later, the hydrogen economy may actually be within reach ‘in a few short years.’
What constitutes the hydrogen economy? According to most analysts, the hydrogen economy is “an economy that relies on hydrogen as the commercial fuel that would deliver a substantial fraction of a nation’s energy and services.”[i] That sounds straightforward, but there are numerous routes by which hydrogen can deliver energy. Some paths involve producing hydrogen and using it directly as fuel in a combustion engine or fuel cell. Other paths make derivatives from hydrogen that are used as fuels. Current hydrogen production would provide only 2.3% of the world’s energy requirements so an 8X increase in production would be needed to reach 20%.
Routes to Hydrogen Economy
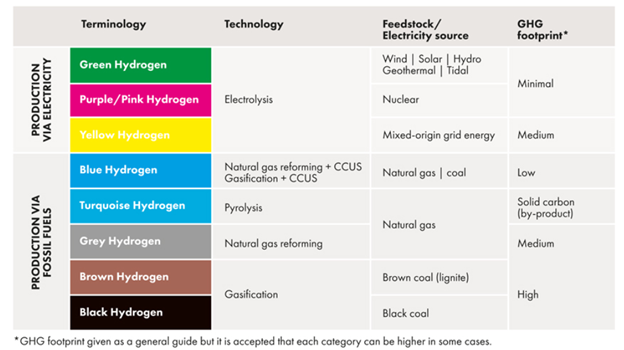
Underlying all the paths to a hydrogen economy are processes for producing hydrogen. Hydrogen, although colorless, has come to be referred to as a particular ‘color’ of hydrogen that depends on the process used to produce it. Colors are assigned based on the feedstock and ‘carbon intensity’ (“CI”) of the production process, i.e. how much CO2 is emitted per kg of hydrogen produced. A sophisticated model, called GREET[ii], developed at Argonne Labs, is used to calculate CI. GREET combines numerous measured properties and process features along with user-designated assumptions to provide a CI value for a particular pathway.
The oldest and most widely used hydrogen production methods start with fossil fuels coal or natural gas, and are labeled as black or grey, since they are considered the least environmentally friendly and have high CI values. (CI is scored like golf; low scores are better) At the other end of the hydrogen spectrum are those processes that rely on renewable energy and involve no carbon directly such as electrolysis of water using electricity from wind, solar, or hydroelectric generation, which are considered green. Those processes produce hydrogen with very low CI scores. In between are numerous variations and combinations that include those that use biomass as feed, nuclear energy, biological processes, or integrate any process with carbon capture and storage (“CCS”) to reduce CI. There are even processes that have negative CI scores, i.e. their net effect is to remove CO2 from the environment. So-called blue hydrogen is hydrogen produced from natural gas using conventional steam methane reforming (“SMR”) with the capture of CO2 for storage.
Figure 1. The Hydrogen Color Spectrum from Global Energy Infrastructure.[i] The spectrum is arranged such that processes with high CI scores are at the bottom and processes with low CI scores are at the top. Low CI scores correspond to lower emissions of CO2.
Impact of Blue Hydrogen
Natural gas continues to replace coal in power plants as a more environmentally friendly substitute due to its lower CI, fewer contaminants, and much less ash. Burning natural gas emits almost 45 percent less carbon dioxide than does burning coal while producing the same amount of energy. Coal combustion typically produces 5-15% by weight ash as well while natural gas combustion produces only miniscule amounts of ash. From 2005 to 2019, according to the U.S. Energy Information Administration[ii], coal use declined from 50% to 23% of the fuel used for electricity generation. As a result, CO2 emissions in the US declined by 13% from 2005 to 2019 and 65 percent of the reduction in CO2 emissions is attributed to the replacement of coal with natural gas to generate electricity.
Carbon capture technologies were developed to remove CO2 from chemical process streams and power plant flue gas. Most of these processes react the CO2 in the flue gas with an absorbent and collect it for storage or other uses. For coal gasification, CCS reduces the CO2 emissions from 287 to 105 gCO2/MJ.[iii] SMR for hydrogen production generates less CO2 than coal gasification at 76 gCO2/MJ and integrating a CCS unit with SMR to produce blue hydrogen reduces the CO2 emissions from the plant further to 40 gCO2/MJ emitted, although some argue that greatly increased methane emissions offset most of the gain from CCS.[iv]
There is no free lunch, however, as the additional CCS processing reduces the overall process efficiency of an integrated combined cycle natural gas-powered plant by about 5 points, from 43% to 38%.[v] A blue hydrogen installation that replaces a conventional coal-fired plant with a gas-fired SMR + CCS plant could reduce the CO2 emissions produced by as much as 86%. Where low emissions electricity is available to produce oxygen, the use of autothermal reforming (ATR) can reduce CO2 emissions even further since the higher concentration of CO2 in the flue gas makes CO2 capture more efficient.
Carbon Capture – Key to Blue Hydrogen
Blue hydrogen relies on carbon capture and storage technology. There are currently 41 operational CCS facilities worldwide, seven of which make blue hydrogen for ammonia used in fertilizer production. CCS has been operational with a capacity to recover about 64 Mtpa of CO2, with 80% of that recovered from natural gas at the wellhead and used for enhanced oil recovery.[vi]
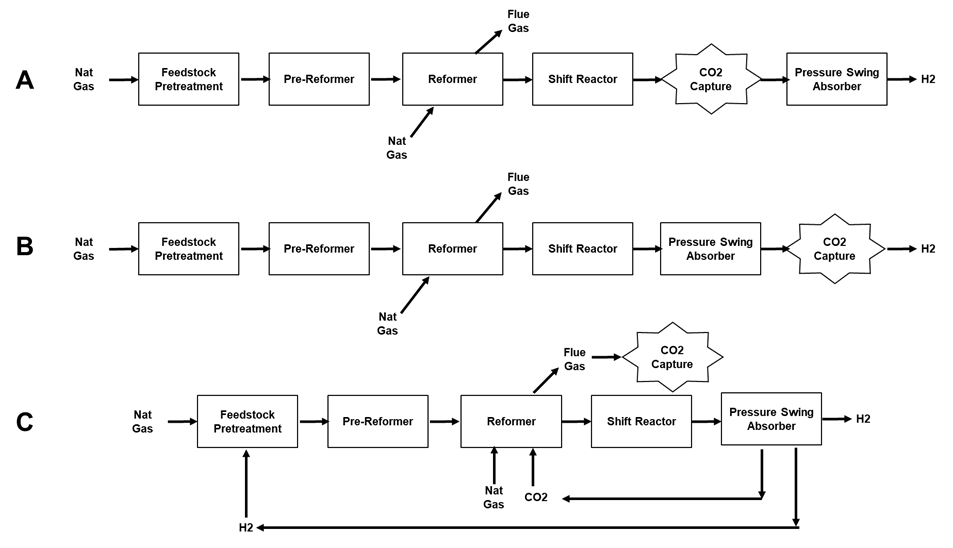
In a typical SMR plant there are 3 process streams from which CO2 could be captured as shown in Figure 2: A) from the shifted syngas, B) from the pressure swing adsorption retentate, or C) from the reformer flue gas.
Figure 2. Options for Capturing CO2 from a Steam Methane Reforming Process.
There are several means of capturing CO2. The most common is chemical absorption using a solvent (MDEA, methyldiethanolamine) that reversibly binds the CO2 that can be released later by heating. Another is cryogenic separation in which the gas mixture is cooled until it liquefies and then distilled into separate components. According to a 2017 IEA report[i], CO2 capture processes increase the capital cost by 18 to 79% while capturing 56-90% of the CO2. Vendors offer systems that can capture as much as 99% of the CO2 contained in a PSA retentate or shift product stream, although about 40% of the CO2 generated in the process would still be emitted in the flue gas.
A conventional SMR facility generates a significant amount of electricity from the steam produced, and any CCS installation reduces electricity production by at least 80%. This reduces revenue and increases net OPEX (operating expenses) which range from 17 to 33% higher with CCS than with SMR alone. A significant advantage of the cryogenic separation process is its insensitivity to the cost of natural gas.[ii] With US natural gas prices ranging from $1.50 to $9.30 per MMBTU over the past 5 years, this is a very attractive feature although it is partially offset by a small requirement to import electricity; the trade-off between natural gas and electricity costs can be pivotal for investors. The major industrial gas companies, in particular, seem to favor cryogenic capture as it takes advantage of their expertise in cryogenic distillation used for air separation.
In 2005, the IPCC reported that adding CCS to an existing natural gas facility increases electricity generation costs by 37% to 69%[iii], although for a new integrated combined cycle plant the differential could be about half as much. More recently, George et al estimated that adding CCS to make a hydrogen plant blue increases capital costs by about 75%.[iv]
Is Blue Hydrogen Viable?
Considerable controversy exists over the role of blue hydrogen in the drive to reduce CO2 emissions. Since blue hydrogen relies on two inputs that are typically non-renewable, i.e. natural gas and electricity, some consider it ‘only a modest improvement’ on current practices. These critics dismiss blue hydrogen as a ‘half-measure’ and are anxious to go for the complete transition to green, possibly zero emissions, hydrogen.
Electrolysis of water is a very popular competitor in the hydrogen production sphere since it is seen as emitting no CO2. However, even those who are dedicated to seeing the hydrogen economy take shape are becoming more circumspect as more is learned about the technical and economic hurdles. The Hydrogen Council, for example, reports in its November 2023 summary that “Estimates of the levelized cost of hydrogen (LCOH) for renewable hydrogen are between 30 and 65 percent higher than those in the October 2022 report.”[v] Nevertheless, in the December 2023 “Hydrogen Insights 2023” the Hydrogen Council projects a 50% drop in the cost of H2 by electrolysis in the next 7 years, and a further 50% cost reduction by 2050.[vi] Technology developers will have a hard time living up to that projection.
The emissions benefits of water electrolysis are being called into question as well. A 2018 report from Delft noted that “the CO2 footprint of blue hydrogen (0.82-1.12 kgCO2 eq / kg H2, [24.6-33.6 kgCO2/MWh]) is comparable with hydrogen produced via electrolysis with renewable electricity sources (0.92-1.13 kg CO2 eq./kg H2, [27.6-33.9 kgCO2/MWh]).”[vii]
Realism
In 2022 blue hydrogen accounted for only 0.7% of hydrogen produced worldwide while water electrolysis accounted for just 0.03%.[viii] These are not the only candidate technologies for the transition to the hydrogen economy since a variety of other potentially low emissions hydrogen technologies are under development.
Of particular note, is the two-step process being advanced by Raven SR. The Raven process first steam reforms any mixture that contains hydrocarbons – MSW, biomass, food waste, plastics – in a rotating kiln, followed by a higher temperature SMR-type reforming step to produce syngas that is readily shifted to hydrogen. The rotating kiln can accommodate solids and separates the unreactive contaminants like glass, metal, and minerals from the useful hydrocarbons in the process, making it applicable to a wide range of situations and feeds.[ix] With green electricity and renewable feeds such as wood waste the process is fully renewable.
With a long history in coal upgrading, gasification holds promise that it can be adapted successfully to waste feeds like MSW or biomass to produce renewable, low-CO2 hydrogen. There are 3 competing gasification schemes. The staged fixed bed gasifier is a pressurized steam/oxygen blown gasification process suitable for the smallest size range. The dual fluidized bed steam-only gasification process (technically a steam reforming process) is operated at atmospheric pressure and is most suitable for intermediate size applications, especially for the production of hydrogen or FT hydrocarbons. It has the advantage that for syngas utilization processes which can handle 15-20% inert gases (e.g. power generation), pure oxygen is not needed, which allows the use of air in the reformer. For the largest applications, pressurized steam/oxygen gasification in a circulating fluidized bed gasifier is preferred. Either adding a CCS process or a CO2 getter like CaO to the gasification process sharply reduces CO2.
In China, it appears that the choice for hydrogen production has been made – coal gasification with CCS. In numerous publications their scientists report calculations that show the CO2 production rates for coal + CCS are similar to electrolysis. Li et al.[x], for example, write: “the carbon footprint of coal to hydrogen is reduced by 52.34%–74.59% to 4.92–10.90 CO2eq/kg H2 after installing CCS technology, which is close to that of solar electricity-based hydrogen production,” and concludes “therefore, China should promote the development of coal to hydrogen with CCS to meet the growing demand for hydrogen, at least before there is a breakthrough in hydrogen production from renewable electricity.” Fan et al.[xi] calculate that “hydrogen production via renewable energy-based water electrolysis has no cost advantage in most regions, but wind power-based electrolysis in Gansu and photovoltaic power-based electrolysis in Chongqing have the potential to compete with the C2HCCS [coal + CCS] process”. Coal now supplies 69% of China’s electricity and is adding a new coal plant almost every week. To date the implementation of CCS has lagged far behind the buildout of coal plants.
Plasma gasification is another competitor. Electrically heated furnaces, combustion flames, and electric discharges have been considered for high temperature plasma generation. The very high temperatures available in plasma systems (~ 3000 °C) are attractive because they decompose the gas into atoms that recombine to a high H2 content syngas. However, the cost of energy, the requirement for expensive materials, and the difficulty in controlling the gas cooling have severely limited applications to hydrogen production. Tacking on an additional CCS unit would merely drive up costs.
Methane pyrolysis is being advanced as a hydrogen source. The temperatures of methane pyrolysis are typically about 1000-1100°C due to the stability of methane; catalysts reduce the required temperatures to the 500-900 °C range. During the reaction, each mole of methane splits into two molecules of hydrogen and one atom of carbon. When compared to steam methane reforming, pyrolysis of methane produces only half as much hydrogen per CH4. However, the energy input for methane pyrolysis (37 MJ/kg H2) is less than that of SMR followed by water gas shift (82 MJ/kg H2).[xii]
CH4 => C + 2 H2
One advantage of methane pyrolysis over other methane or natural gas hydrogen production technologies is the production of solid carbon instead of CO2. The lack of CO2 emissions makes methane pyrolysis a cleaner and more attractive hydrogen production pathway. Solid carbon can be a valuable product in its own right or could be steamed to generate CO and additional H2. The drawback is that in addition to H2, pyrolysis makes a complex byproduct mixture of hydrocarbons including some tars that present operational issues. If pyrolysis uses a renewable methane source such as biogas, it could be one of the ‘greener’ alternatives.
Prospectus
Blue hydrogen stands out from its competitors in many ways. Most significantly, it is here, now, and available as a very valuable tool by which to reduce CO2 emissions. It combines known technologies that have long track records of commercial success at massive scale. Industrial concerns are starting to get seriously involved.
Exxon recently announced an ambitious plan to produce “up to 1 billion cubic feet per day of [blue] hydrogen from natural gas and expect over 98% of the associated CO2 to be captured and safely stored underground.” The blue hydrogen “site would be the largest low-carbon hydrogen project in the world at planned startup in 2027-2028.”[xiii] About 7 million tons of CO2 per year will be captured using Honeywell UOP’s CO2 fractionation and hydrogen purification system.
German energy provider, Onyx Power, announced plans to build a 300 kta blue hydrogen plant in the Port of Rotterdam. The plan includes SMR for hydrogen production, CCS, and storage of the CO2 in depleted offshore gas fields, potentially saving up to 2.5 million tonnes of CO2 per year.
This plant will join Shell’s planned electrolyzer plant being built in the Port of Rotterdam[xiv] to make Rotterdam a hub for both renewable hydrogen and CO2 sequestration. The green hydrogen plant will use power from the Hollandse Kust Noord wind farm and is expected to come on-line in 2025. It will have a 200 MW electrolyzer and produce over 20 ktpa of renewable hydrogen.
Shell and Onyx are part of Dutch consortium ‘H-vision’ that together with The Port of Rotterdam, Royal Vopak, ExxonMobil, Air Liquide, and Deltalinqs represent the entire hydrogen chain – from producers to end users.
The Great Plains Synfuels Plant that has been gasifying coal to hydrogen for ammonia production and collecting CO2 for enhanced oil recovery, is being converted to a natural gas fed blue hydrogen facility and re-branded as the “Great Plains Hydrogen Hub.” Originally built in 1984, it will produce 348,000 tons/yr of blue hydrogen when it becomes commercially operational in 2027, and “the cost of production will be the lowest in the country.”[xv] The $2 bn revision of the plant will utilize ATR and CCS to sequester 3 Mtpa of CO2.
Not every project is going forward as planned, however. Calgary-based Nauticol scrubbed their planned $4 bn blue methanol facility that was expected to include blue hydrogen and CCS.[xvi] A smaller plant is being discussed.
While all of this activity shows that blue hydrogen and its derivatives are beginning to be actualized, there are some targets that hold even more promise. Methane is about 25x more potent than CO2 as a greenhouse gas (GHG). Despite efforts to reduce gas flaring, it still accounts for about 150 billion cubic meters of CO2 globally each year, just a bit less than that produced by agriculture. If this methane could be recovered and converted to blue hydrogen the impact on CO2 emissions would be enormous.
If we embrace blue hydrogen as a logical, practical stepping-stone on the path to a zero CO2 future, maybe Professor Sir John Cadogan’s vision of the hydrogen economy will arrive ‘in a few short years.
About the Author
Dr. Mazanec has been involved in the conversion of methane to hydrogen, fuels, and chemicals for much of his 43 years in the industry. During twenty-one years at SOHIO/BP he invented the Oxygen Transport Membrane process for the in-situ separation of oxygen from air for the production of syngas from natural gas and led an international industrial technical team to develop and scale up the process. He served 9 years as Chief Scientist at Velocys, leading the team developing microchannel processes for natural gas upgrading via steam reforming and FT conversion. Since 2011 Terry has been an independent consultant assisting companies ranging from startups like Anellotech, where he was interim CTO, to global energy and chemicals organizations. Dr. Mazanec has been a Subject Matter Expert at LEC since 2015 and currently serves as a Managing Director. He has authored 20 refereed publications and has been granted more than 75 US Patents as well as numerous international patents. Terry earned a Ph.D. in Inorganic Chemistry from The Ohio State University under the direction of Prof. Devon Meek and conducted postdoctoral research at the University of Illinois at Urbana-Champaign.
About LEC Partners (LEC)
LEC has over 180 experts that can help navigate your bioeconomy needs. If you need assistance with your Hydrogen project(s), please Contact Us.
[i] IEA Tech Report 2017-02, “Techno – Economic Evaluation of SMR Based Standalone (Merchant) Hydrogen Plant with CCS,” https://ieaghg.org/component/content/article/49-publications/technical-reports/784-2017-02-smr-based-h2-plant-with-ccs
[ii] Ibid.
[iii] IPCC Chapter 3,”IPCC Special Report on Carbon dioxide Capture and Storage,” https://www.ipcc.ch/report/carbon-dioxide-capture-and-storage/capture-of-co2/
[iv] George, J. F., “Is blue hydrogen a bridging technology?,” Energy Policy 167 (2022), 113072.
[v] The Hydrogen Council, “Global Hydrogen Flows – 2023 Update – Considerations for evolving global hydrogen trade,” November 2023, https://hydrogencouncil.com/en/global-hydrogen-flows-2023-update/
[vi] The Hydrogen Council, “Hydrogen Insights 2023 December Update,” https://hydrogencouncil.com/en/hydrogen-insights-2023-december-update/
[vii] Delft, CE Delft, “Feasibility study into blue hydrogen – Technical, economic & sustainability analysis,” https://cedelft.eu/publications/feasibility-study-into-blue-hydrogen/
[viii] George, J. F., et al,. op cit.
[x] Li, et al, “The carbon footprint and cost of coal-based hydrogen production with and without carbon capture and storage technology in China,” J Cleaner Production, 362, 2022, 132514; https://doi.org/10.1016/j.jclepro.2022.132514 .
[xi] Fan et al, “A levelized cost of hydrogen (LCOH) comparison of coal-to-hydrogen with CCS and water electrolysis powered by renewable energy in China,” Energy 242, 2022, 123003; . https://doi.org/10.1016/j.energy.2021.123003 .
[xii] Korányi, Tamás I., et al. “Recent Advances in Methane Pyrolysis: Turquoise Hydrogen with Solid Carbon Production.” Energies 15.17 (2022): 6342.
[xiii] Exxon website, 30-Jan-2023, “Low-carbon hydrogen: Fueling our Baytown facilities and our net-zero ambition,” https://corporate.exxonmobil.com/news/viewpoints/low-carbon-hydrogen
[xiv] Shell website, “Shell to start building Europe’s largest renewable hydrogen plant”, 7-Jul-2022, https://www.shell.com/media/news-and-media-releases/2022/shell-to-start-building-europes-largest-renewable-hydrogen-plant.html
[xv] MHA Nation Partnering with Bakken Energy and Mitsubishi Power on Great Plains Hydrogen Hub, February 9, 2022, https://www.bakkenenergy.com/mha-nation-partnering-with-bakken-energy-and-mitsubishi-power-on-great-plains-hydrogen-hub/ .
[xvi] https://www.cbc.ca/news/canada/edmonton/calgary-energy-firm-backs-away-from-proposed-4b-northern-alberta-methanol-plant-1.6739176
[i] https://globalenergyinfrastructure.com/articles/2021/03-march/hydrogen-data-telling-a-story/
[ii] EIA; https://www.eia.gov/todayinenergy/detail.php?id=48296
[iii] The Hydrogen Council, “Hydrogen decarbonization pathways – A life-cycle assessment,” January 2021, https://hydrogencouncil.com/en/hydrogen-decarbonization-pathways/
[iv] Howarth, RW, Jacobson, MZ, “How Green is Blue Hydrogen?” Energy Sci Eng, 2021, 1676-1687.
[v] Hendriks, C.A., Blok, K., Turkenburg, W.C. (1989). “The Recovery of Carbon Dioxide from Power Plants.” In: Okken, P.A., Swart, R.J., Zwerver, S. (eds) Climate and Energy: The Feasibility of Controlling CO2 Emissions. Springer, Dordrecht. https://doi.org/10.1007/978-94-009-0485-9_9 .
[vi] Global CCS Institute, “Global Status of CCS 2023,” https://status23.globalccsinstitute.com/ .
[i] Nehrir, M. H., Wang, C., “Fuel Cells”, in “Electric Renewable Energy Systems”, Academic Press, 2016 92-113, https://www.sciencedirect.com/science/article/pii/B9780128044483000062 .
[ii] GREET stands for Greenhouse Gases, Regulated Emissions, and Energy Use in Transportation, https://www.energy.gov/eere/bioenergy/articles/greet-greenhouse-gases-regulated-emissions-and-energy-use-transportation .
Have some questions?
Not sure where to start?
Let's start a conversation. We're here to help you navigate
the bioeconomy with confidence.