Biochar for Energy Storage Applications
By Dr. Kent Goeking LEC Partners
Biochar is the charcoal-like carbon product obtained when biomass is heated in an oxygen-deficient environment, i.e. pyrolysis. Biochar is well known for its agriculture amendment value for increasing crop yields, and as a form of activated carbon in water filtration and toxin remediation applications. It is also important as a carbon sequestration strategy as recognized by the UN Intergovernmental Panel on Climate Change (IPCC).
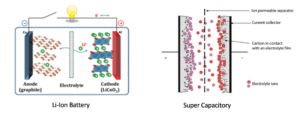
The feedstock used, and the quality produced of biochars can vary widely but it is possible through well-controlled pyrolysis technologies to create biochars that can be considered engineerable in their properties and serve as a new sustainable carbon platform. Such carbons would be suitable for more sophisticated applications like replacing the graphite and activated carbon electrode materials in high-demand energy storage devices including Li-Ion batteries, and supercapacitors.
Figure 1 – Schematics of Li-Ion battery and Supercapacitor designs
A typical automotive EV battery requires about 50 kg of graphite for its Li-ion battery. That graphite comes from either natural forms that are mined, or synthetically made from petroleum coke. Neither source is sustainable or capable of meeting future graphite demand. Furthermore, natural graphite and synthetic graphite sell for $5000-$10,000/ton, whereas biochar can be produced at a significantly lower price.
High-Performance Energy Storage Devices
High-performance batteries and supercapacitors using biochar as the source for the carbon electrode material have been produced by many university and commercial labs. These devices consistently outperform commercially available graphite-based energy storage devices. However, like its cousin graphene, the question remains as to whether this high-quality biochar can be produced at scale outside the lab. That requires biochar production technologies that are geared more towards the production of an engineerable substrate carbon and not just for agricultural usage which is considerably more forgiving.
Most biochar production technologies today were either developed primarily for biofuel (syngas) production with biochar as a non-optimized by-product or were targeted at high-volume production of agriculture-quality biochar through continuous processes. In order to take advantage of biochar’s engineering potential, there needs to be a production technique that can produce lab-quality biochar at scale, where material quality is the primary driver, not biochar or syngas volume.
The production of biochar for electronic applications is a multi-step process. The important aspects of biochar for electrode applications are its microporosity, the morphology of the graphite sheets, and heteroatom doping. The process starts with the selection of feedstock which can determine initial, carbon content, porosity, and naturally occurring dopants as well as how sustainable the production can be at higher volumes. The production of consistent, uniform-quality biochar is then best accomplished using a batch production technique that can deliver more uniform temperature distributions across the feedstock during conversion, and complete the carbonization process without significant dependence on feedstock particle size or initial moisture levels.
Thai Carbon Co. Ltd. in Thailand is currently developing such a technical grade biochar using technology developed over 10 years by Biochar Now in Colorado, USA. The batch pyrolysis system is based on a ring furnace design, as opposed to more common retort style furnaces, which allows for auto thermal internal heating and superior thermal uniformity and control. During pyrolysis hemicellulose and cellulose break down at lower temperatures with a mixture of endothermic and exothermic reactions interacting with each other to form a difficult-to-control thermal environment. After cracking these biomass components into their syngas and char constituents, the remaining lignin component then provides a more controllable fuel/feedstock for precision thermal control.
While in this thermal controllable state, the entire feedstock batch (typically 1000 kg woody biomass at <20% moisture) is allowed to soak at a specific setpoint temperature, typically 675°C, for several hours. This allows for the full conversion of the feedstock into carbon and removes most if not all of the volatile tars and other compounds that take time to volatize out of deep pores in the feedstock. The soak time and temperature can also be adjusted to increase the level of crystallization into graphite phases or to encourage more amorphous carbon structures. Typically, as produced biochars will have high carbon contents (>85-90%), high surface areas (~300 m2/g), and good electrical conductivity.
Issues with current electrode materials
In current Li-Ion battery designs, the commercial graphite used as the anode material offers a low theoretical capacity of 372 mA h/g and limited rate performance. The processes of intercalation and deintercalation of Li-ions between the graphite layers are the key to Li-ion operation but if a Li-ion battery is charged too quickly, intercalation may not smoothly occur.
Instead of penetrating the graphite matrix, the Li-ions can aggregate on the surface of the anode, resulting in an effect called ‘plating’ that can impair or destroy the cell. That places a practical limit on how fast a Li-ion can be charged. In addition to plating, rapid charging results in a build-up of chemical products from side reactions in the graphite pores, causing irreversible expansion of the graphite and further impairing performance.
Furthermore, the graphite industry is under pressure to reduce pollution levels. Current purification processes are mainly done in China and require a large quantity of chemicals that have a negative environmental impact.
Current supercapacitor electrode materials also suffer a discrepancy between theoretical and actual capacitance achieved which should be a direct result between the specific capacitance and specific surface area (SSA) AC electrodes. Supercapacitors with high SSA electrodes of around 3000m2/g often demonstrate a relatively small capacitance indicating that not all pores are effective during charge accumulation. Hence, even though the SSA in supercapacitors is an important parameter when it comes to performance, what appears to be of greater importance is the pore size distribution.
Three critical parameters for biochar to enhance performance have been identified in the literature for both supercapacitors and Li-ion batteries:
Porosity enhancement
Results for Bamboo biochar with subsequent heat treatment with KOH for enhanced porosity supercapacitor resulted in the activated bamboo biochar with more than 3000 m2/g of surface area with uniform small pore size most with pore sizes below 2nm. Typical activated carbon has a range of pores with sizes up to 50nm.
Figure 2 – Illustration of macro-, meso- and microporosity of biochar
The prominence of micropores resulted in a high specific capacitance of more than 300F/g compared to typical commercial supercapacitor performance of 203F/g with high-quality activated carbon. Furthermore, the capacitance retention was 92% after 3000 cycles. Li-ion batteries also display enhanced mechanical properties for better cycling.
Morphology Control
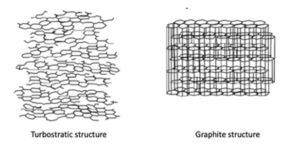
Li-ion anode performance increases with disordered turbostatic (hard) carbon morphology. This allows for easier insertion and removal of Li ions as opposed to the regimented graphite crystalline structure.
Figure 3 – Different morphologies for biochar
However, for supercapacitor performance, it is best to lose the graphite structure and reduce the structure to be more amorphous which will increase supercapacitor performance.
Heteroatom self-doping
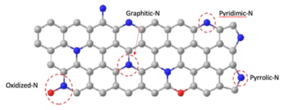
The introduction of heteroatoms (an atom other than carbon in the ring of heterocyclic compounds) into the carbon matrix of the biochar material is another effective approach to improving the super capacitance performance. Many biochars naturally contain significant self-doping levels N, P, O and S.
Figure 4 – Different heteroatom doping sites for biochar
The capacitance performance of a nitrogen-containing bamboo biochar material activated with KOH and the results show that although the as-synthesized material had a relatively low surface area of 221 m2/g, it could achieve exceptional specific capacitances of 297 and 284 F/g. This superior capacitance is mainly attributed to the pseudo-capacitance arising from the redox reactions of the nitrogen and oxygen functionalities (the material contained 10 wt% oxygen and 8 wt% nitrogen). Furthermore, the electrodes demonstrated excellent cycling stability; only 3% capacitance fading was observed after 10 000 cycles, demonstrating good conductivity and rapid charge propagation in both acidic and basic electrolytes.
For Li-ion batteries, the doping of electron-rich N atoms into the graphite carbon matrix of biochar also introduces more chemically active defects. This not only enhances the electronic conductivity but also provides more available active sites for Li+ adsorption, resulting in high Li+ storage capacity. When used as an anode material for Li-ion batteries, the nitrogen-doped biochar delivered a high reversible capability of 505 mA /g at 0.1°C, 1.36 times higher than the theoretical capacity of graphite. Furthermore, the nitrogen-doped biochar demonstrated a high-rate capability of 190 mA h/g at 10°C (1°C = 372 mA h/g). Developing carbon-based materials with a larger interlayer distance caused by heteroatom incorporation will also help to increase the diffusion rate of Li ions and speed the recharging of the cell.
Conclusion
The experimental literature reviewed to date indicates that biochars from a variety of feedstocks are highly suitable as both supercapacitor electrodes and Li-ion battery anode materials. The performance can be increased by enhancing the pore densities, changing the morphology, and doping the materials with heteroatoms. The key will be upscaling the lab-produced biochars into industrial-scale production while preserving quality. Biochar can become an important sustainable engineered carbon for helping solve future energy storage requirements.
About the Author:
Dr. Kent Goeking has over 30 years of experience in developing and deploying next-generation business and technology solutions. He is very active in the fields of pyrolysis (both woody biomass and plastics) and precision fermentation currently assisting several major companies in developing advanced biotech capabilities as they migrate to biobased products from their historical petrochemical and agricultural commodity markets. He is a Project Director at LEC Partners, overseeing matters involving Pyrolysis, Biochar, Fermentation, Bioproducts, Synthetic Biology, and projects in the SE Asia/India markets.
Have some questions?
Not sure where to start?
Let's start a conversation. We're here to help you navigate
the bioeconomy with confidence.